Introduction
Electrodeposition is important in many different disciplines such as microelectronics, nanobiosystems and solar energy conversion. Surface properties of a substrate are modified to enhance electrical conductivity, improve corrosion resistance and heat tolerance. The morphology of the surface substrate determines the quality of the deposition, creating the need for monitoring the process at nanoscale.
In this application note, the deposition and dissolution of copper nanoparticles onto a gold surface was clearly observed in situ using a Park NX10 AFM. Current-voltage (CV) curves were simultaneously acquired using a potentiostat.
Experimental
An electrochemical cell, manufactured by Park Systems, was used in the experiment. This cell is an electronic circuit with three different electrodes connected to a potentiostat. An electric field was applied to transmit electrons from the working electrode to the ions in the copper solution, initiating chemical reactions. The amount of applied voltage determines whether a reduction or oxidation reaction occurs. Cyclic voltammetry (CV) was applied to study these chemical reactions in the solution. This measurement is followed by linear sweep voltammetry to stimulate copper deposition on a gold (111) surface and subsequent dissolution of the particles back into the solution. Simultaneously, AFM surface topology images are acquired in true non-contact mode. Copper nanoparticles were quantified using Park XEI software.
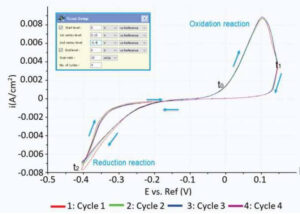
Figure 1. Cyclic voltammogram showing the regimes for oxidation and reduction reactions.
Results
Figure 1 shows the cyclic voltammogram of the deposition and dissolution of copper particles. Oxidation reactions occur in the positive peak, where the negative peak indicates deposition of copper on the substrate. This redox process was confirmed with AFM measurements. Figure 2A shows a reference AFM image of the gold surface before electrochemical reactions are applied. An image of the gold surface after deposition is depicted in Figure 2B. All copper particles larger than 5.5 nm were quantified and colored. When an oxidation reaction is initiated, copper particles start to dissolute back into the solution and the surface becomes free from copper again (Figure 2C).
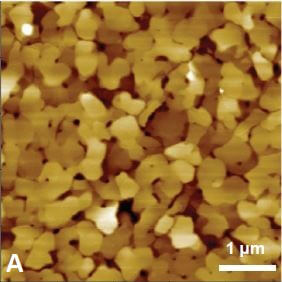
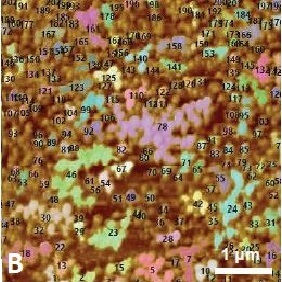
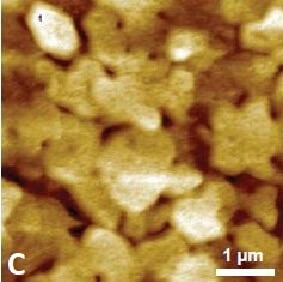
Figure 2. (A) Gold (111) surface before copper deposition. (B) Surface of the gold substrate after deposition. Copper particles are identified and quantified. (C) Almost all copper is dissolved back into the solution.
In this study, electrochemical atomic force microscopy was applied successfully during in situ measurements of copper electrodeposition. This technique enables researchers to monitor morphological changes and to obtain better insights in their electrochemical experiments.
Download the application note for a full description of the experimental set up and detailed results.
