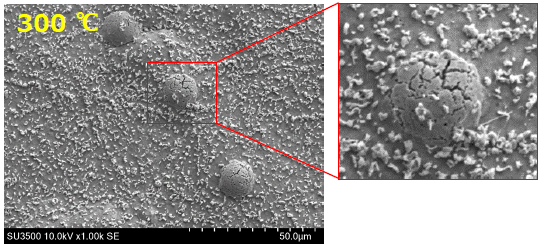
Plating is a coating method in which a thin layer of metal is deposited on a substrate. This process is applied to improve aesthetics or to alter material properties such as corrosion resistance, friction, strength or conductivity. Zinc is a common plating material to provide protection against oxidation and corrosion. The substrate is immersed into an electrolyte solution containing zinc ions and an electrical current is applied to deposit ions on the substrates surface. A metallic coating is now covering the surface and prevents degeneration of the substrate due to corrosion. To be effective, the film layer should be non-porous, i.e. no microcracks or gaps are allowed. This application note demonstrates the effect of increasing temperatures on the quality of zinc plated surfaces.
To read the entire document, download the application note.