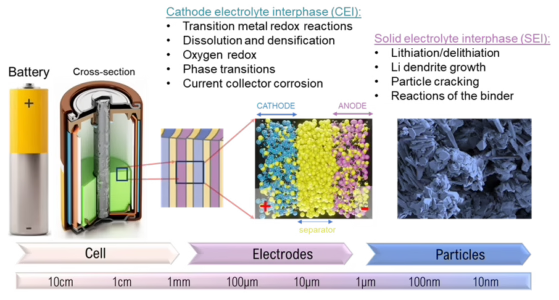
Batteries are complex, multi-layered systems with many layers and interfaces that need to be understood. In a typical battery cell, there is a separator/electrolyte at the center, sandwiched between a cathode and anode, with current collectors on either end. During charge and discharge, ions will flow across these layers, interacting with many components including organic and inorganic species, solvents, and active materials.
As a result, interfacial layers form at the electrode-electrolyte interface, with chemical and structural consequences on battery stability. At the cathode electrolyte interphase (CEI), changes can happen such as transition metal redox reactions and particle dissolution, while in the solid electrolyte interphase (SEI), lithiation and delithiation processes can lead to particle cracking, dendrite growth, and harmful side reactions with the binder (Figure 1). Battery materials must be analyzed in detail to understand how and where interfacial products are formed to improve material performance for long-term device operation. Specifically, understanding the chemical composition, distribution, and morphology of electrode-electrolyte interfaces is necessary to prepare well-dispersed, stable, high-capacity electrodes.
Learn More?
Please click on ‘Request Application Note’ and we will send you the full application note ‘Mosaic Mapping for Analysis of Heterogeneous Battery Degradation’.
