Probing Interfaces in Fuel Cell Electrodes by XPS and HAXPES
Fuel cells are one of the fastest-growing alternative energy sources and the most promising technologies for market penetration in automotive applications and different consumer areas. Fuel cells consist of an anode, a cathode, and an electrolyte that allows either positively-charged hydrogen ions or negatively-charged hydroxyls to move between the two sides of the fuel cell. The catalyst layers on the anode and cathode consists of the catalyst material and ionomer, which provides ionic conductivity. The chemistry of the catalyst layer has the most significant impact on overall fuel cell performance.
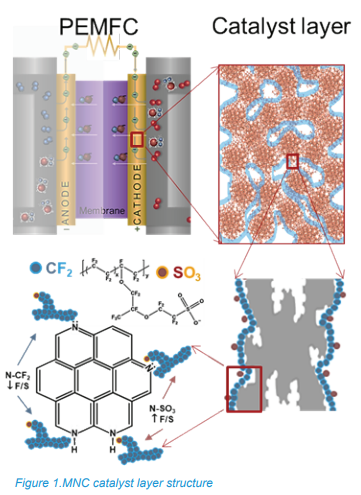
Efforts to replace Pt-based oxygen reduction reaction (ORR) electrocatalysts with platinum-group metal (PGM) PGM-free alternatives, particularly transition metal−nitrogen− carbon (MNC) electrocatalysts, have advanced significantly. In MNC electrodes, the distribution of active sites that facilitate the oxygen reduction reaction, in “morphology of the ionomer” and structure of ionomer-catalyst interface within the cathode catalyst layers is critical. As shown in Figure 1, in the cathode catalyst layer, the catalyst is mixed with the ionomer Nafion, which interacts with the chemical groups on the catalyst’s surface. Nitrogen chemistry is central to understanding the types and abundance of active sites present within the electrode, while carbon chemistry captures ionomer (Nafion)-catalyst morphology within the catalyst layer.
The application of XPS to study catalyst layers and their structure has become a standard analytical approach due to its chemical specificity, rich qualitative and quantitative information, and ease of access. While surface sensitivity of XPS is an important attribute, in some cases, the depth of analysis of XPS is not sufficient to analyze deeper gradients and interfaces without first sputter etching the sample surface. An alternative to sputter-etching the sample is Hard X-ray Photoelectron Spectroscopy (HAXPES), in which high energy hard X-rays (energy > 5kV) are used to provide a signal from depths of analysis three or more times than that of soft X-rays used on conventional XPS systems.
This note describes an application of a laboratory-based instrument, the PHI Quantes, equipped with two scanning microprobe monochromated X-ray sources, Al Kα (1486.6 eV) and Cr Kα (5414.8 eV), to fuel cell electrodes, thus enabling both traditional XPS and HAXPES experiments in the same instrument. Combining both soft and hard X-ray analyses, we can better understand the composition and structure at the surface and buried interfaces.
Please click on ‘Request Application Note’ and we will send you the full application note ‘Probing Interfaces in Fuel Cell Electrodes by XPS and HAXPES’.