X-ray Fluorescence Analyzers
♦ XGT-9000 | X-ray Analytical Microscope (Micro-XRF)
♦ XGT-9000SL | X-ray Analytical Microscope Super Large Chamber Model
♦ MESA-50 | X-Ray Fluorescence Analyzer
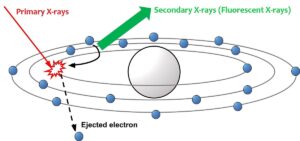
When a molecule is irradiated with x rays, it may emit secondary fluorescent x-rays. This happens when the incident x rays expels an electron from one of the orbitals. The hole is filled by an electron from a higher energy orbital, which then emits the extra energy in several ways, one of which are fluorescent x rays.
The energy difference between incident and emitted x-rays characterizes the fluorescent atom. This is why x ray fluorescence is an excellent analytical technique to determine elemental composition. We offer a range of x ray fluorescence analyzers which are suited for applications from materials science, biomedical and pharmaceutical analysis to geology and engine wear analysis.
When a primary x ray expels an electron from an atomic orbital, the hole will be filled by electrons from higher energy orbitals. This means that the fluorescent x rays can have different energies, depending on their orbital or origin. Thus, one element can show different x ray fluorescent peaks, originating from different orbitals, which together form a fingerprint for that element.
The energy of emitted x rays is independent of the molecular environment of the element. As the interaction of x rays with heavier atoms is more efficient, the technique is more sensitive to heavier elements. However, it can still be used for light elements down to beryllium (atomic number 4). Two different x ray fluorescence methodologies are used for heavy or light elements: Energy Dispersive XRF measures the energies of emitted x rays and is used for elements from Sodium to Uranium, while Wavelength Dispersive XRF analyzes the wavelength of the emitted x rays and is used for the lighter elements.
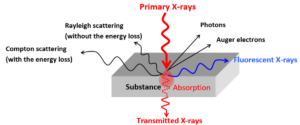
X ray fluorescence is a non-destructive technique for fast chemical analysis. It is used in a wide range of applications, to investigate for example metals or polymers in areas like forensics, archeology or environmental science, but also in geology or mining. The resolution can be from 3 mm down to 10 micron in an x ray Analytical Microscope, and results can provide qualitative and quantitative data. Furthermore, x ray fluorescence works in different sample types, from liquids to solids and everything in between.
ST Instruments provides a range of x-ray fluorescence analyzers by Horiba scientific to cover all kinds of applications.